01 Feb 2024
Professor Heechae Choi from the Department of Chemistry, School of Science at Xi’an Jiaotong-Liverpool University (XJTLU) revealed the secret of the Vehicle fuel cell cathode reactions by solving the problems of the discrepancies between the device phenomena and the classical electrochemistry theory for the first time. The paper titled "Switching Electric Double Layer Potential by Phase Structure Control for Advanced Oxygen Reduction Reaction of Cobalt@Nitrogen Doped Carbon Core-Shell" was published in the chemistry journal Small (Wiley, with an impact factor of 13.3).
Fuel cell cathodes in most of the commercial hydrogen vehicles use metal/carbon composites as the electrocatalysts of oxygen reduction reaction (ORR) (Figure 1). The development of the metal/carbon for ORR electrocatalysis has been made mostly via countless, exhaustive and expensive trial-and-error experiments because the classic electrochemistry theory is not particularly applicable to this modern technology.
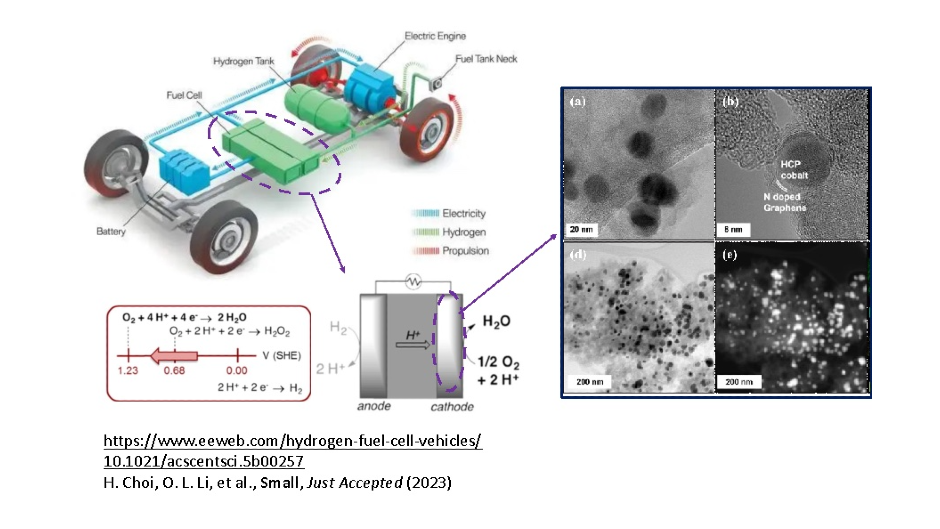
Figure 1. Schemes of the vehicle fuel cell, cathode reactions, and the transmission electron microscopy image of a cobalt/carbon composite nanoparticle.
Prof. Heechae Choi and his theory group combined classical electrodynamics theory and quantum mechanics calculations (Figure2), to create a modified version of the electrical double layer (EDL) theory model. In his theoretical predictions, the phase transformation of cobalt core nanoparticle inside the carbon shell, switches electrostatic potential in the EDL near the nanoparticle surface.
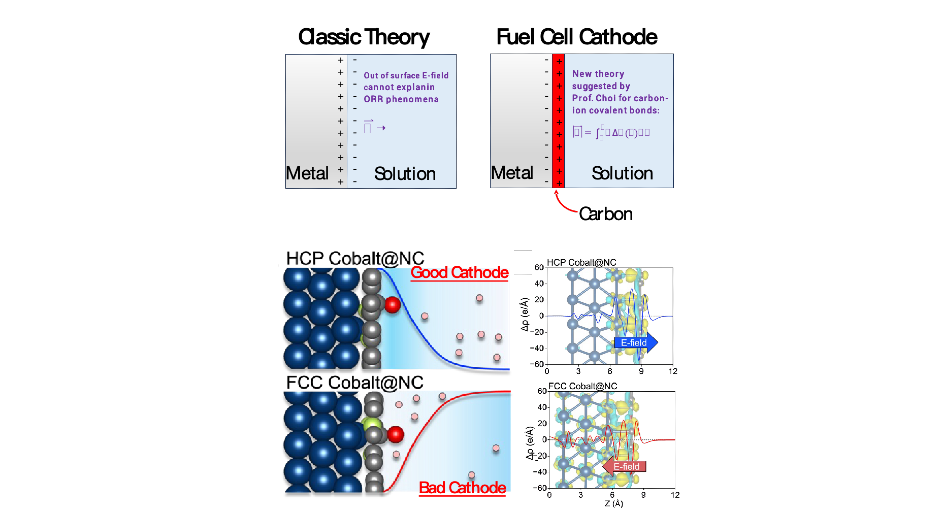
Figure 2. Comparison of the classic theory in electrochemistry from a textbook and the new theory suggested by the Department of Chemistry, School of Science, XJTLU (top), and the predictions for cobalt@carbon core-shell nanoparticles.
To prove Prof. Choi’s new theory and predictions, Prof. Helena Oi Lun Li (Pusan National University, ‘PNU’ hereinafter), a renowned scientist in plasma synthesis field and her research group from South Korea, tested the electrocatalytic ORR activity of cobalt@carbon core-shell nanoparticles for two different phases of cobalt core, FCC and HCP (Figure 3).
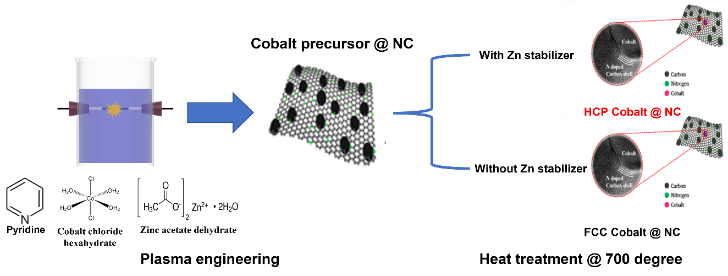
Figure 3. Plasma syntheses of cobalt@carbon nanoparticles
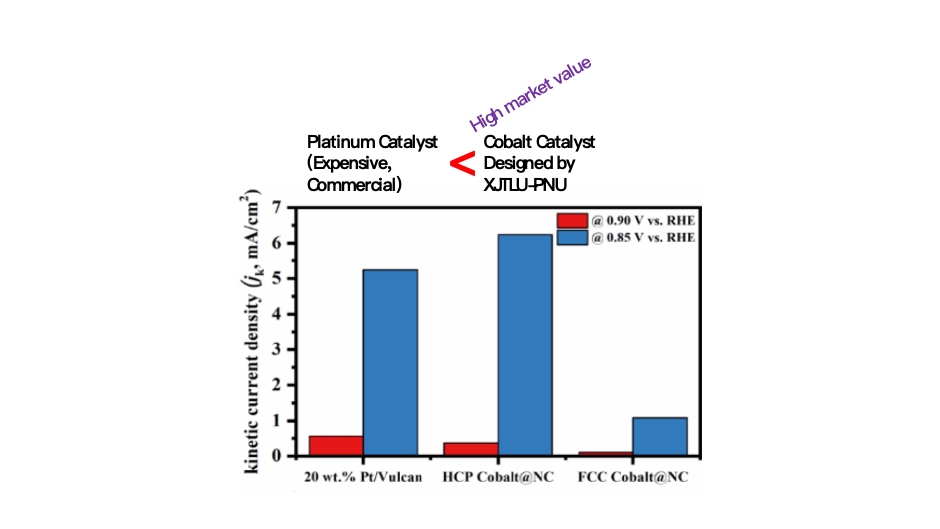
Figure 4. Comparisons of current densities of a commercial platinumbased material (Pt/Vulcan) and Cobalt@Carbon developed by XJTLU-PNU (H. Choi, O. L. Li, Small)
Prof. Choi’s new theoretical predictions and Prof. Li’s plasma synthesis technique demonstrated that the cheaper cobalt metal can produce better efficiency in vehicle fuel cells than the current commercial platinum based expensive materials (Figure 4).
This valuable research output was a byproduct of a budding and strong partnership between the School of Science at XJLTU, China and the School of Materials Science & Engineering at PNU, South Korea, after the two Schools signed an MoU in July, 2023 (Figure 5).

Figure 5. Group photos of XJTLU-PNU taken on July 3rd, 2023 in Korea (top) and on Oct. 28th, 2023 in China (bottom).
Professor John Moraros, Dean for the School of Science, XJTLU stated: “This joint international research work by Prof. Choi (XJTLU, China) and Prof. Li (PNU, South Korea) is very significant in the field of electrochemistry theory. It represents the first of many collaborative efforts by the team that aim to shine a light on the discovery and developments of renewable energy materials to help improve the welfare and wellbeing of mankind.”
01 Feb 2024








