06 Mar 2024
Recently,The XPad (XJTLU Peptide and Drug) group, a collaboration between Dr Antony Kam from the Department of Biological Sciences, School of Science, XJTLU, and Dr Shining Loo from the Wisdom Lake Academy of Pharmacy, together with the team of Professor Liu Chuan-Fa and Professor James P. Tam from the Nanyang Technological University, Singapore, published the research results entitled "Ultrafast Biomimetic Oxidative Folding of Cysteine-rich Peptides and Microproteins in Organic Solvents" in Angewandte Chemie.
The research introduces a novel ultrafast biomimetic approach for folding cysteine-rich peptides and microproteins in organic solvents, mimicking natural protein folding mechanisms to achieve rapid and precise folding. By leveraging this innovative strategy, the study demonstrates the instantaneous and high-yield production of correctly folded microproteins, showcasing its potential for advancing peptide synthesis and bioengineering applications.

Click here to read the original text
Dr Antony Kam said that Cysteine-rich peptides, typically consisting of less than 50 amino acids and characterized by the presence of 3-5 disulfide bonds, are compact microproteins renowned for their remarkable stability. These peptides/microproteins can be synthetically generated in laboratories using solid-phase peptide synthesis, a technique pioneered by Nobel Laureate Robert Bruce Merrifield, and can be further customized to produce innovative drug candidates through epitope grafting.
“The presence of disulfide bridges in these peptides/microproteins is crucial for maintaining their proper folded structure, stability, and biological functionality. However, a significant challenge in the chemical synthesis of these cysteine-rich peptides lies in the oxidative folding process, where multiple disulfide bonds must form coordinately to achieve the correct folded conformation.”
According to Dr Kam, While the conventional oxidative folding of these peptides typically takes place in aqueous buffered solutions, this study aims to overcome the limitations associated with this method by introducing a novel organic oxidative folding approach that enhances the rate of disulfide bond formation in polar aprotic solvents. This innovative strategy significantly expedites the folding process, achieving an impressive acceleration rate compared to traditional aqueous methods.
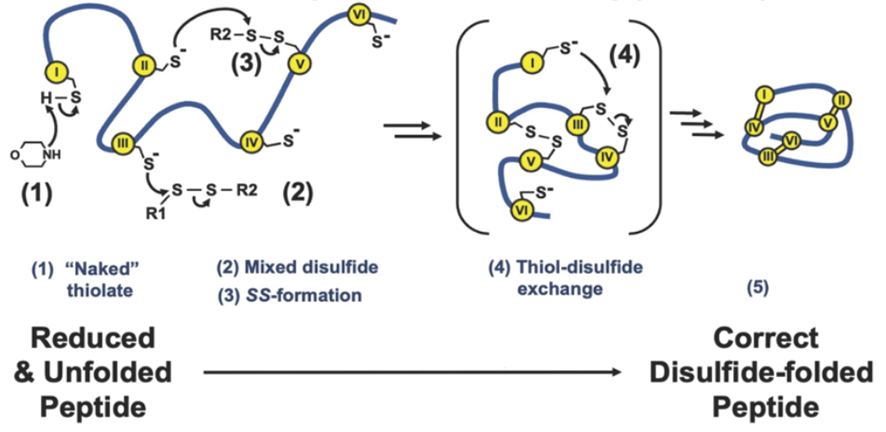
The efficacy and versatility of this novel folding technique are demonstrated by 15 diverse cysteine-rich peptides, encompassing variations in hydrophobicity, length (ranging from 14 to 58 residues), and number of disulfide bonds (2-5 bonds). This organic oxidative folding method showcases its potential for rapid and efficient folding of cysteine-rich peptides within seconds, thereby advancing peptide engineering and drug development.
Dr Antony Kam joined the Department of Biological Sciences, School of Science at Xi’an Jiaotong-Liverpool University as an Assistant Professor in 2023. He graduated with a Bachelor of Pharmacy (Honours) degree (2010) and a Ph.D. in Pharmacy (2015) from the Faculty of Pharmacy, University of Sydney, Australia, focusing on natural product pharmacology. After graduation, he was appointed first as a Research Fellow, and later, promoted to Senior Research Fellow, at the School of Biological Sciences, Nanyang Technological University, Singapore, under the supervision of a world-renowned peptide chemist, Professor James P. Tam (2014-2023).
The XPad research group specializes in the fields of chemical biology, synthetic biology, and molecular pharmacology, with a particular focus on expanding the therapeutic applications of peptides. Please scan the QR code or click here for further information regarding ongoing research activities conducted by the XPad research group.

XPad: XJTLU Peptide and Drug research group
06 Mar 2024








