Introduction
This platform integrates artificial intelligence and experimental methods to cover the entire early-stage innovative drug R&D process—from target identification, lead compound design, and screening to compound synthesis, property analysis, and structural optimization—providing an efficient, systematic, and integrated solution. Supported by the university’s high-performance computing platform with a CPU-GPU heterogeneous architecture and 648 TB parallel storage, it enables computationally intensive tasks such as deep learning, molecular simulation, and virtual screening. It provides robust computational power for Computer-Aided Drug Design (CADD) and AI-Driven Drug Discovery (AIDD), enabling large-scale molecular modeling, virtual screening, and AI model training to significantly enhance precision and R&D efficiency.
Research Direction and Collaboration Areas
- Deep learning-based molecular generation methods
- Structure-based drug design and discovery
- Mass spectrometry-based compound screening
- Separation and pharmacological and efficacy evaluation of active ingredients in traditional Chinese medicine
- Drug discovery for metabolic diseases & neurodegenerative diseases
- Optimization of drug synthesis routes based on continuous flow technology
Core Techniques

Bioaffinity mass spectrometry

Magnetic Bead-based Biomolecular Affinity Separation Technology

AI-driven Molecular Generation
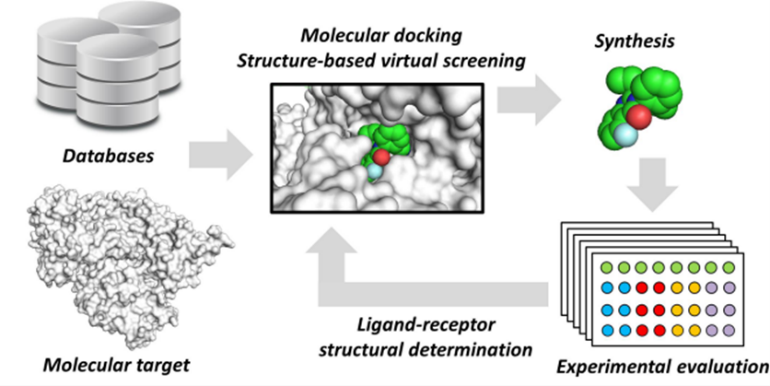
Structure-based Drug Design
Supporting Resources
- XJTLU High-Performance Computing Platform:
Supports GPU heterogeneous computing and 648 TB parallel storage, enabling AI model training, virtual screening, and molecular simulation for efficient CADD and AIDD research.
- In vitro and in vivo Drug Screening and Validation Platform:
Includes cell activity assays, organoids, affinity mass spectrometry (ASMS), SPR molecular interaction analysis, and mouse/rat/C. elegans models for efficacy and safety evaluation.
- Small Molecule Design and Synthesis Module:
Integrates compound synthesis, property analysis, and lead optimization to support early-stage drug R&D.
- Target and Virtual Screening Tools:
Equipped with AI-assisted algorithms such as NAPU-bagging to improve target identification and candidate screening efficiency.
Research Projects
- NSFC Young Scientist Project (2024–2026): Deep learning-based prediction and optimization of non-coding sequences in mRNA therapeutics
- NSFC Young Scientist Project (2023–2025): Total synthesis and druggability study of macrocyclic depsipeptide Verucopeptin to overcome tumor resistance
- Jiangsu Provincial General Natural Science Project (2024–2026): Mechanistic study on repairing infarcted myocardium using combinations of stem cell-derived extracellular vesicles
- Jiangsu Provincial NSFC Young Scientist Project (2023–2026): Optimization of the “cold start” issue in deep learning-based drug screening models for target proteins
- SIP Major Innovation Platform Construction Project (2022–2025): Innovation Platform for Chronic Disease Study
Patents
- CN106674175B: Method for synthesizing coumarin compounds
- CN108314727B: Membrane-type metalloproteinase inhibitor and its applications
- CN111705037A: 3D co-culture model of fibroblasts and cancer cells, its preparation and applications
- CN109260228A: A compound for tumor therapy
- WO2019/134526A1: Metalloproteinase inhibitor and related pharmaceutical compositions and applications
Publications
- Journal of Medicinal Chemistry, 2025, 68 (2), 1473-1482, Targeted Degradation of HCV Polymerase by GalNAc-Conjugated ApTACs for Pan-Genotypic Antiviral Therapy with High Resistance Barriers
- Nature Communications, 2025, 16, 4683, DNA nanoflower Oligo-PROTAC for targeted degradation of FUS to treat neurodegenerative diseases
- Science Bulletin, 2024, 69 (13), 2122-2135, An engineered DNA aptamer-based PROTAC for precise therapy of p53-R175H hotspot mutant-driven cancer
- Nucleic Acids Research, 2024, 52 (D1), D194-D202, m6A-Atlas v2.0: updated resources for unraveling the N6-methyladenosine (m6A) epitranscriptome among multiple species
- Angewandte Chemie – International Edition, 2024, 64 (12), e202422023, Conditional Relay Activation of Theranostic Prodrug by Pretargeting Bioorthogonal Trigger and Fluorescence-Guided Visible Light Irradiation
- Angewandte Chemie – International Edition, 2024, 63 (14), e202317789, Ultrafast Biomimetic Oxidative Folding of Cysteine-rich Peptides and Microproteins in Organic Solvents
- Acta Pharmaceutica Sinica B, 2024, 14 (7), 653-666, Broad-spectrum ginsentides are principal bioactives in unraveling the cure-all effects of ginseng
- Cell Genomics, 2024, 4 (12), 100718, AI techniques have facilitated the understanding of epitranscriptome distribution
- ACS Materials Letters, 2024, 6 (9), 3993-4001, Structural Ordering of Interfacially Assembled Silk Fibroin-Like Peptides via Robust Intermolecular Hydrogen-Bonding Networks





Introduction
This platform focuses on the discovery and validation of novel biomarkers. Leveraging AI-powered omics technologies, it adopts a systems biology perspective to comprehensively interpret molecular changes related to health and disease. The platform provides strong support for the entire drug R&D process, clinical diagnostics, and precision medicine. Additionally, it explores translational applications of biomarkers, including the development of innovative detection technologies and high-throughput screening of lead compounds, offering new strategies for biomarker-based clinical applications and drug discovery.
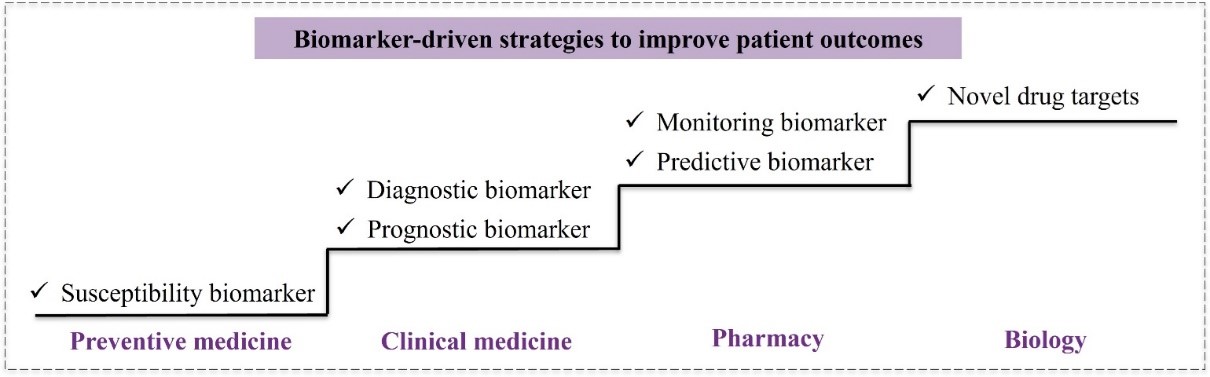
Research Direction and Collaboration Areas
- High-throughput omics and algorithm-driven biomarker mining and disease prediction modeling
- AI-powered integration of multi-dimensional data to support drug development and patient stratification
- Development of non-invasive detection technologies empowered by functional materials for improved sensitivity and convenience
Core Techniques
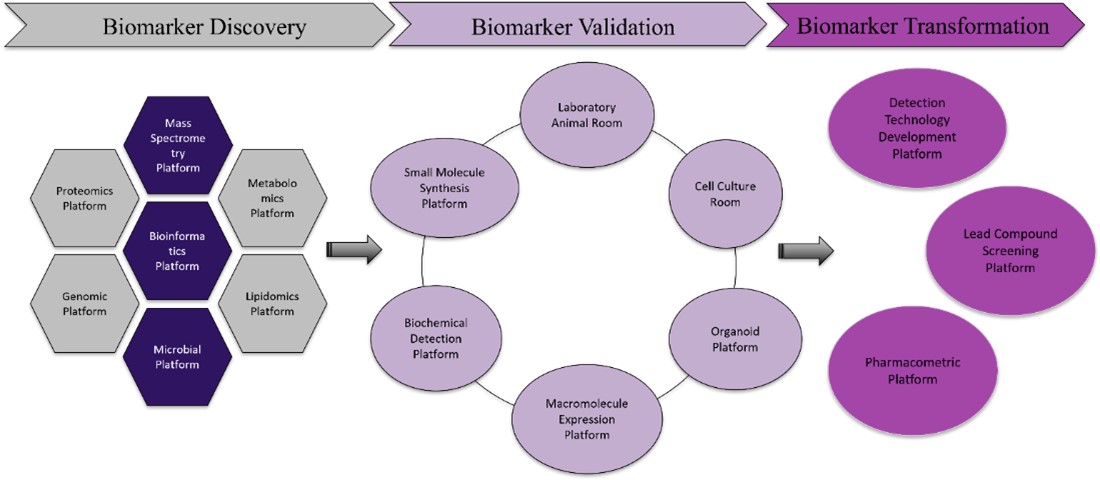

Supporting Resources
The Academy of Pharmacy has established a high-caliber research team and an internationally advanced interdisciplinary platform covering the full research chain from biomarker discovery to validation and translation. This significantly enhances the efficiency of biomarker development for major diseases such as cardiovascular and oncological disorders, providing robust technical support for precision medicine and new drug development.
- Mass Spectrometry Analysis Platform:
Equipped with state-of-the-art instruments, including high-resolution mass spectrometers (Qtrap series, QToF), triple quadrupole quantitative MS, and 400/600 MHz NMR systems, alongside customized omics pipelines that greatly improve biomarker discovery efficiency and data reliability.
- Bioinformatics Analysis Platform:
Utilizes machine learning-driven omics workflows and multi-dimensional data integration to provide powerful computational support for biomarker screening.
- Biological Validation Platform:
Features Biocore molecular interaction systems and Seahorse metabolic analyzers, complemented by standardized cell culture facilities, an SPF-grade animal center, and organoid-based drug evaluation systems. Together, these form a complete “molecule–cell–animal” validation framework to accelerate biomarker translation and drug development.
Research Projects
- NSFC Young Scientists Fund (2025–2027): Study on binding characteristics, mechanisms, and regulation of multivalent recognition in genome-wide single-molecule detection
- NSFC Young Scientists Fund (2025–2027): Mechanistic study on dimethylguanidino valeric acid-induced β-cell dysfunction and early progression of type 2 diabetes
- SIP Major Platform Construction Project (2022–2025): Innovation Platform for Chronic Disease Study
- SIP Major Platform Construction Project (2020–2023): Macro-Molecular Characterization Analysis Centre
Patents
- CN116270704A: A pharmaceutical composition for inhibiting SOD1 gene expression and its applications
Publications
- Nucleic Acids Research, 2024, 52 (9), 4830-4842, m6ACali: machine learning-powered calibration for accurate m6A detection in MeRIP-Seq
- Chemical Engineering Journal, 2024, 497, 154553, A porous elastomer with a cavity array for three-dimensional plantar force sensing
- Cell Reports, 2024, 43 (7), 114402, Translocational attenuation mediated by the PERK-SRP14 axis is a protective mechanism of unfolded protein response






Introduction
Our goal is to establish an innovative, professional, and comprehensive research platform, providing the ability to further optimize the current drug delivery systems, specifically develop suitable drug delivery systems for small molecule drugs and macromolecule biopharmaceuticals, and design corresponding new drug formulations and delivery strategies, to meet different clinical needs and improve drug stability and bioavailability.
Research Direction and Collaboration Areas
The focus is on the development of new materials, new formulations, and new strategies for drug delivery, including two main directions: (micro-/nano-) drug delivery systems and novel drug formulations.
- Research on (micro-/nano) Drug Delivery Systems:
- Development of lipid-based drug delivery systems
- Development of extracellular vesicles-based drug delivery systems
- Development of drug delivery systems based on proteins and polymers
- Development of virus-based drug delivery systems
- Research on New Drug Formulations:
- High Bioavailability Oral Administration
- Transdermal Administration
- Nasal Administration
- Targeted Drug Delivery
- Long-Acting/Sustained-Release Formulation
- Novel Cocrystal Drug
Core Techniques
- Smart-responsive materials for controlled and targeted delivery
- Long-acting and oral delivery technologies for protein/peptide drugs
- Nucleic acid drug delivery using viral, exosome, and novel lipid nanocarriers
Research Projects
- Jiangsu Provincial Basic Research Program (Youth Project, 2024–2027): Study on novel gene therapy strategy using extracellular vesicle-encapsulated adeno-associated virus to overcome pre-existing neutralizing antibodies
- Jiangsu Provincial Key Laboratory Construction Project (2024–2027): Key (Construction) Laboratory for Jiangsu Province Universities: Nanoformulation for Cell Therapy
Patents
- CN118255866A: A crystalline form of lanreotide salt, its preparation method, and applications
- CN109920939A: Solvent, method, and application for preparing high-performance metal halide perovskite thin films
- CN108314727B: Membrane-type metalloproteinase inhibitor protein and its applications
- CN111705037A: A 3D co-culture model of fibroblasts and cancer cells, its preparation method, and applications
- CN109260228A: A complex for tumor treatment
- WO2019/134526A1: Membrane-type metalloproteinase inhibitor protein and pharmaceutical or drug compositions containing it, and their respective uses
Publications
- Chemical Science, 2024, 15 (31), 12291-12300, Intensified electrochemiluminescence and photoluminescence via supramolecular anion recognition interactions






Introduction
The platform focuses on the development and optimization of continuous flow systems, carrying out innovative drug design, synthesis, analysis, optimization, and screening. It aims to establish automated continuous flow systems with an integrated combination of hardware and software (such as equipment, automated control, analytical methods, scale-up strategies, etc.), and to build various application scenario databases, assisting in the full-chain innovative drug research and development. The platform is equipped with high-end reactor systems and analytical equipments to support the design, validation, continuous optimization, and industrial exploration of various small molecule drug compounds and biopharmaceuticals (such as antibody drugs and conjugate drugs).
Research Direction and Collaboration Areas
- Discovery of novel drugs (small molecules and biologics) and development of continuous flow processes
- Construction and optimization of continuous flow equipment systems
- Application of automated control and online monitoring systems to achieve real-time supervision and product quality consistency
Core Techniques

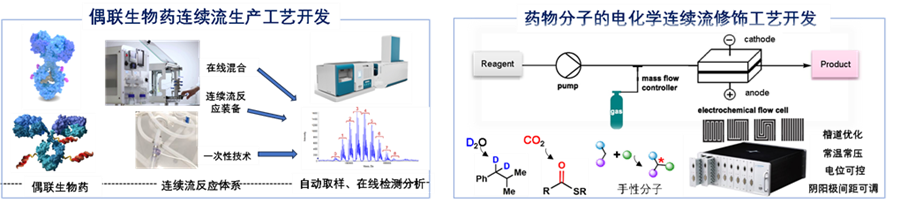
Research Projects
- NSFC Young Scientists Fund (2022–2024): Construction of an electrocatalytic epoxidation system for alkenes based on acyl functionalization strategy
Publications
- Chemistry – An Asian Journal, 2025, e202500086, MXene-Induced Construction of SnS2 Nano-Arrays with Sulfur Vacancies for High-Efficiency Photocatalytic CO2 Reduction
- ChemCatChem, 2024, 16 (13), e202301534, Electro-Oxidation of Alkenes: A Green Approach Towards Functionalized Oxygenates
- Journal of the American Chemical Society, 2022, 144 (33), 15185-15192, Highly Selective Electrocatalytic Oxidation of Amines to Nitriles Assisted by Water Oxidation on Metal-Doped α-Ni(OH)2
- Green Chemistry, 2020, 22 (21), 7543-7551, Highly selective electrocatalytic oxidation of benzyl C–H using water as safe and sustainable oxygen source






Introduction
This platform provides more clinically relevant research models for preclinical drug efficacy, drug intake, drug safety evaluation, and cell therapy, to support the drug development and regenerative medicine research needs of biopharmaceutical research institutions and enterprises. The institute has a research team engaged in the design and development of organoid technology, and has established patient-derived breast cancer organoids, pancreatic ductal adenocarcinoma organoids, and conducted preclinical studies on drug efficacy, drug intake, and drug safety. It has also established pluripotent stem cell-derived islet organoids and small intestinal organoids to explore innovative treatment options for diabetes and inflammatory bowel disease, achieving excellent therapeutic effects.
Research Direction and Collaboration Areas
- Construction of patient-derived organoid disease models and drug screening
- Establishment of organoid and pluripotent stem cell biobanks and technical service collaboration
- Development of organoid-on-chip and high-throughput drug screening systems
Core Techniques
- The design and development of patient-derived tumor organoids with independent property rights for the preclinical evaluation of drug efficacy, drug intake, and drug safety.

Cancer Res (2023) 83 (17): 2924-2937
- Conducting research on drug resistance using patient-derived pancreatic ductal adenocarcinoma organoids.

Data not yet published
- Designing and optimizing a 3D culture-based system for the directed differentiation of pluripotent stem cells towards functional islet-like organoids, with implications for innovative diabetes therapy.

Research Projects
- NSFC Young Scientists Fund (2025-2027): Mechanistic and synergistic study of extracellular vesicles from human pluripotent stem cells and their derived cardiomyocytes in promoting myocardial repair after infarction
- Jiangsu Provincial Universities Natural Science General Program (2024–2026): Mechanistic study on the combinational repair of infarcted myocardium by extracellular vesicles derived from multiple stem cell lineages
Patents
- CN114716693A: A reprogramming-responsive smart hydrogel material, its preparation method, and applications
- CN111705037A: A 3D co-culture model of fibroblasts and cancer cells, its preparation method, and applications
Publications
- Cell Regeneration, 2025, 14 (12), Cell reprogramming: methods, mechanisms and applications
- Nature Biomedical Engineering, 2025, 9 (2), 215-233, Potent prophylactic cancer vaccines harnessing surface antigens shared by tumour cells and induced pluripotent stem cells
- Advanced Materials, 2024, 36 (22), 2211609, Cell-Reprogramming-Inspired Dynamically Responsive Hydrogel Boosts the Induction of Pluripotency via Phase-Separated Biomolecular Condensates
- Cancer Research, 2023, 83 (17), 2924-2937, Nanoparticle-Based Combination Therapy Enhances Fulvestrant Efficacy and Overcomes Tumor Resistance in ER-Positive Breast Cancer




Introduction
This platform can be utilized for pharmacokinetic (PK) and pharmacodynamic (PD) modeling, building comprehensive databases, and conducting model analysis. Researchers can simulate and forecast the behavior of drugs in vivo through this platform, optimize clinical dosages, support clinical trial design, and provide decision support to help expedite the drug development process.
Research Direction and Collaboration Areas
- Preclinical study design and pharmacokinetic/pharmacodynamic (PK-PD) data analysis
- Clinical pharmacology consulting and multi-stage clinical design support
- Population pharmacokinetic (POPPK) and mechanism-based PK-PD and disease model development
- Exposure–response (E-R) analysis and optimization of clinical protocols and dosing strategies
- AI-assisted model construction and regulatory document preparation
Core Techniques
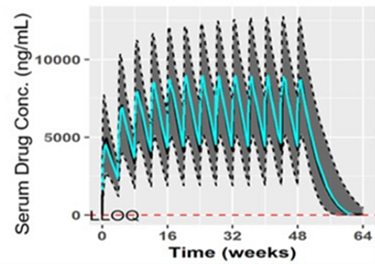
Modeling and simulation of drug concentration–time curves
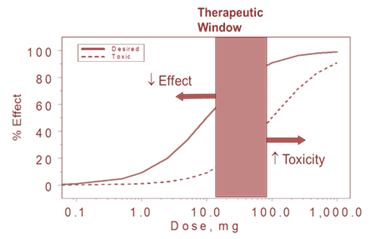
Prediction and sensitivity analysis of dose–response relationships

Regulatory, Clinical Pharmacology, Quality, and Clinical Operation
Publications
- BMC Medical Research Methodology, 2025, 25, 111, An investigation into the impact of temporality on COVID-19 infection and mortality predictions: New perspective based on Shapley values
- BMC Public Health, 2025, 25, 488, Spatio-temporal modelling of extreme low birth rates in U.S. counties
- One Health, 2025, 20, 101038, Spatio-temporal pattern and risk factors of HIV/AIDS prevalence in Zhejiang, China, from 2005 to 2022 using R-INLA
- bioRxiv, 2025, 635451, How Does Sampling Affect the AI Prediction Accuracy of Peptides’ Physicochemical Properties?
- Mathematics, 2024, 12 (3), 388, A Conceptual Framework for Quantifying the Robustness of a Regression-Based Causal Inference in Observational Study
- Sociological Methods & Research, 2024, 51 (4), The probability of a robust inference for external validity: a probabilistic generalizability index for randomized experiments
- Communications in Statistics: Simulation and Computation, 2024, 53 (10), 4685-4702, Robust variable selection via nonconcave penalties with an upgraded parsimonious dynamic covariance modeling




Introduction
This platform aims to assist researchers in understanding regulatory requirements, optimizing research and development management, mastering research and development logic, extending drug life cycles, and promoting and advancing scientific regulation through in-depth exploration of the entire process of innovative drug research and development, technology transfer, large-scale production, market use, and post-market research.
Research Direction and Collaboration Areas
- AI-enabled regulatory science research and decision support for pharmaceuticals
- Analysis of domestic and international regulatory frameworks and technical requirements for innovative drug registration
- Formulation of registration strategies and decision-making assessment at key development stages
- Management of pharmaceutical development strategies and regulatory communications with authorities
- Risk-based post-marketing variation and lifecycle management
- Specialized training and customized corporate courses on new drug R&D and regulatory compliance
Previous Events
The platform has successfully organized several specialized training sessions on innovative drug regulation, focusing on key review points, submission strategies, and critical clinical milestones. These events brought together former senior reviewers from institutions such as the FDA and CDE to help enterprises and R&D teams align with regulatory trends and practical approaches.
Session 1 | Innovative Drug R&D Strategies and Evaluation Considerations
- Review and approval processes and accelerated pathways
- Pharmaceutical research and early-stage clinical development strategies
- Clinical data assessment and benefit–risk evaluation for market authorization
Session 2 | Considerations and Case Studies in Innovative Drug Development
- Top-level design and registration essentials in drug development
- Pharmaceutical variations during clinical stages and conditional approval strategies
- In-depth analysis of representative innovative drug cases
The platform continues to offer training on regulatory compliance, policy interpretation, and professional consultation, establishing a solid support system for the regulatory science of innovative drugs.








In October 2024, to better align with the needs of industrial R&D, XJTLU Wisdom Lake Academy of Pharmacy strategically deployed seven technology platforms focused on cutting-edge technologies in drug research and development. Leveraging the University’s resource integration advantages, the Academy continues to expand and deepen collaborations with the industry. The platform teams have established diverse and extensive partnerships with over 40 enterprises, including Hengrui Pharmaceuticals, Ascentage Pharma, and Corning Continuous Manufacturing Technology. In addition, four joint laboratories have been established with industry partners to collaboratively advance project development, while jointly cultivating urgently needed international and interdisciplinary high-level talents for the biopharmaceutical sector.